Assessing Arginase activity through an easy-to-use immunoassay now possible!
May 22, 2023 2025-06-05 16:48Assessing Arginase activity through an easy-to-use immunoassay now possible!
Assessing Arginase activity through an easy-to-use immunoassay now possible!
Free L-Arginine (Arg) is provided by the diet, de novo synthesis, and protein turnover. De novo synthesis is important enough not to consider Arg as an essential amino acid. However, in some circumstances – e.g. early development, infection, and inflammation – de novo synthesis is not sufficient and requires dietary Arg, which is thus classified as a conditionally-essential amino acid. Whatever the source, Arg can be metabolized through different degrading pathways which include i) the nitric oxide synthase (NOS) pathway providing Citrulline and NO, as well as ii) the well-known Arginase pathway leading to the production of Urea and L-Ornithine (Orn).
In mammals, through a limited amount of Arg and the concomitant production of Orn, elevated Arginase activity has been linked to dysfunctions and pathologies of the cardiovascular system, kidneys, and central nervous system as well as to dysfunction of the immune system and cancer (1). In the context of oncology, Arginase – types 1 & 2 – can be expressed by tumor cells or immune cell subsets including Myeloid Derived Suppressive Cells (MDSCs), and, through its metabolic activity, creates an immunosuppressive milieu. As to restore an effective anti-tumor immune response, blocking the Arginase pathway has been considered an attractive immunotherapeutic strategy (2).
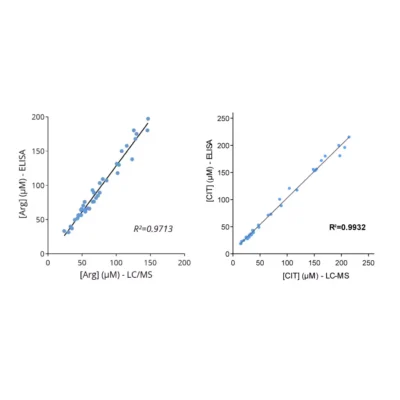
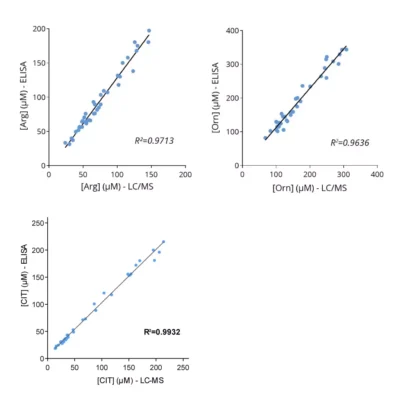
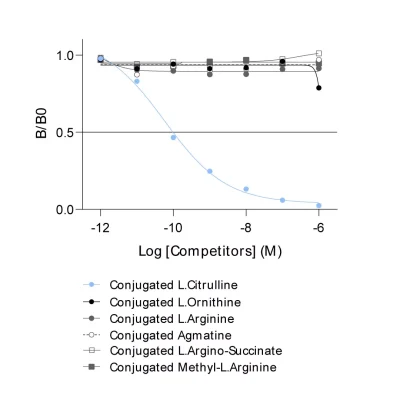
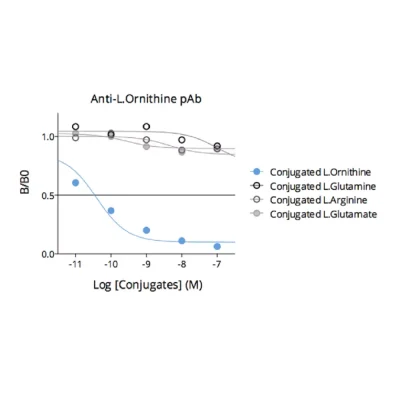
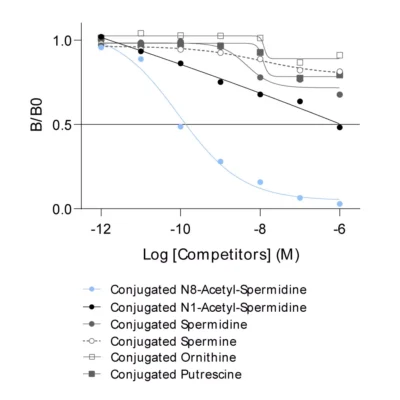
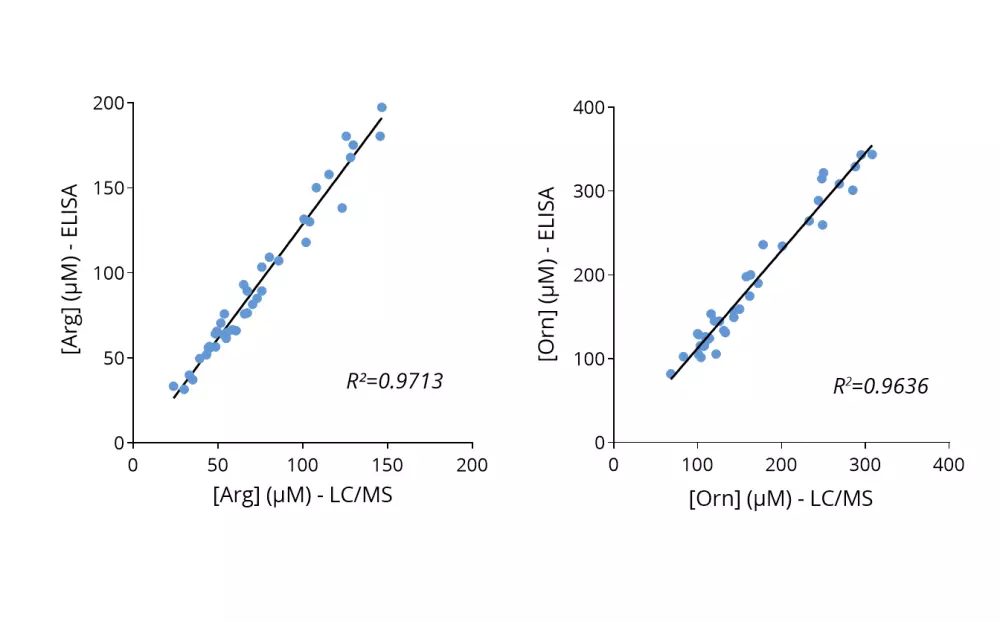
Given the importance of this metabolic pathway in pathophysiological processes and in particular in immune regulation, we have developed and validated a robust and easy-to-use immunoassay (#ISE-0410R) for the simultaneous detection and quantification of both Arg and Orn in plasma / serum from multiple species. Immuno-assay characteristics have been first validated with the comparison of both Arg and Orn levels quantified through conventional mass spectrometry and our immune assay on the same plasma samples (Figure 1), thus validating our methodology.
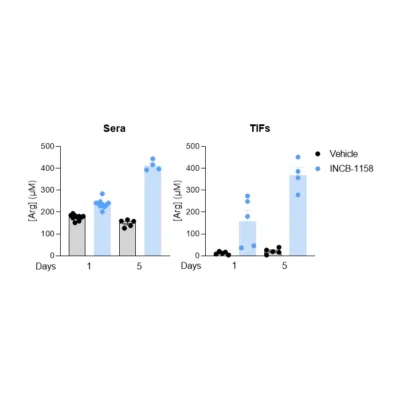
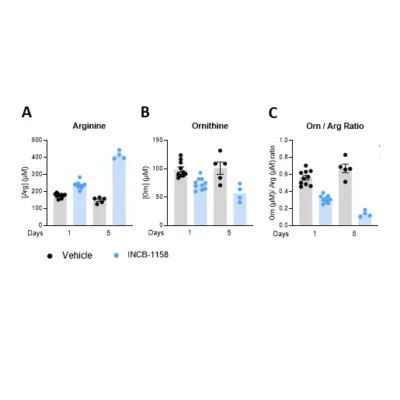
Also, using a syngeneic MC38 mouse colorectal tumor model, we evaluated, using our immunoassay, Arg and Orn serum levels upon animal treatment with a single or five consecutive injections of the prototypical Arginase inhibitor – INCB-1158 – or its respective vehicle solution. This assay revealed the ability of INCB-1158 to restore the level of Arg and limit, at the same time, Orn production. This dataset confirms the robustness of our ELISA kits to quantify Orn and Arg levels as well as the accuracy of Orn to Arg ratio as a surrogate of Arginase activity.
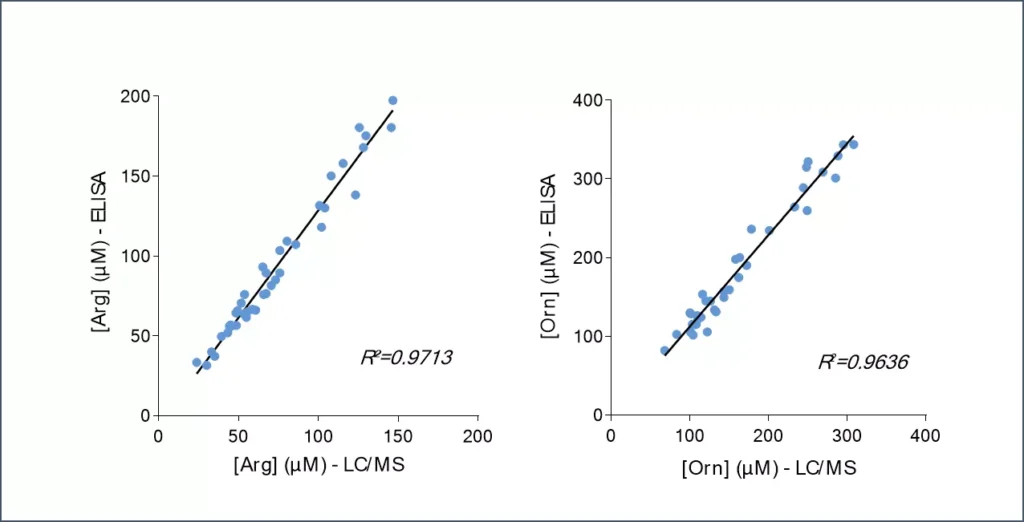
Figure 1 – Cross-validation of Arg and Orn ELISA and LC/MS data in human plasma samples. Arg and Orn were quantified in human plasma samples from healthy subjects using #IS-I-0400R and #IS-I-1000R ELISA kits or by LC/MS. Correlation study confirmed the accuracy of the immunoassay.Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
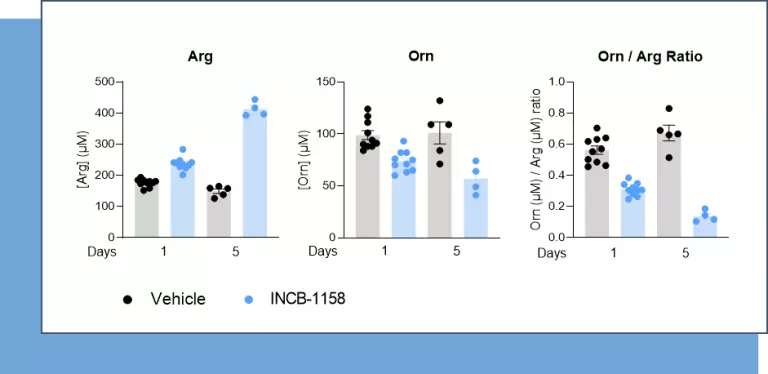
Figure 2 – The arginase inhibitor INCB-1158 restores Arg level while limiting Orn production in sera of MC38-tumor bearing mouse model. C57BL/6J mice were subcutaneously inoculated with MC38 colorectal cancer cell line and treated orally with 100mg/kg of INCB-1158 for one or five consecutive days. Sera were collected after each treatment period and subjected to Arg and Orn level quantification using #IS-I-0400R and #IS-I-1000R ELISA kits, respectively. Orn to Arg ratio was then calculated as a surrogate of Arginase activity.
(1) https://pubmed.ncbi.nlm.nih.gov/29412048/ (2) https://pubmed.ncbi.nlm.nih.gov/35316752/
Global Arginine Bioavailability ratio (GABR) ELISA pack
€1499€1797